
Let's Review: Element Identity & Reactivity
Quiz
•
Chemistry, Science
•
6th - 8th Grade
•
Medium
Standards-aligned

Victoria Gandy
Used 5+ times
FREE Resource
Enhance your content
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
An atom of which element has 37 protons, 48 neutrons and 1 valence electron?
Neon (Ne)
Carbon (C)
Rubidium (Rb)
Sodium (Na)
Tags
NGSS.HS-PS1-1
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
When trying to identify an unknown element, a scientist determines what other elements reacts with chemically. Which property of the unknown element determines the other element it reacts with?
The total number of neutrons in the unknown element
The total number of particles in the nucleus of the unknown element
The number of protons in the nucleus of the unknown element
The number of valence electrons in the unknown element
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
The atoms of a certain element each contain 9 protons and 7 valence electrons. Which statement correctly identifies this element and describes the chemical reactivity?
The element is oxygen, and is highly reactive.
The element is fluorine, and is highly reactive.
The element is oxygen, and is not very reactive.
The element is fluorine, and is not very reactive.
4.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which model represents the most reactive atom?
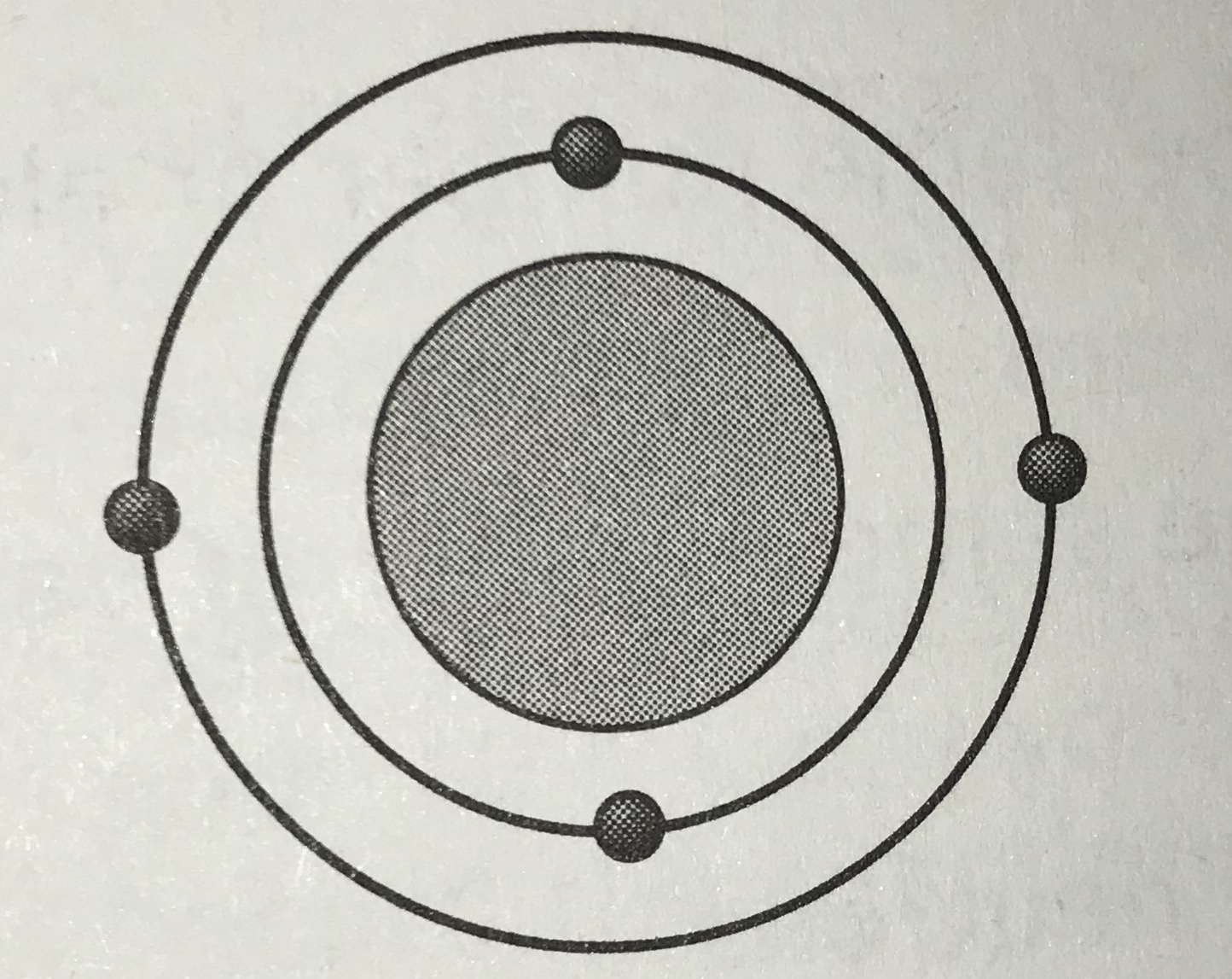
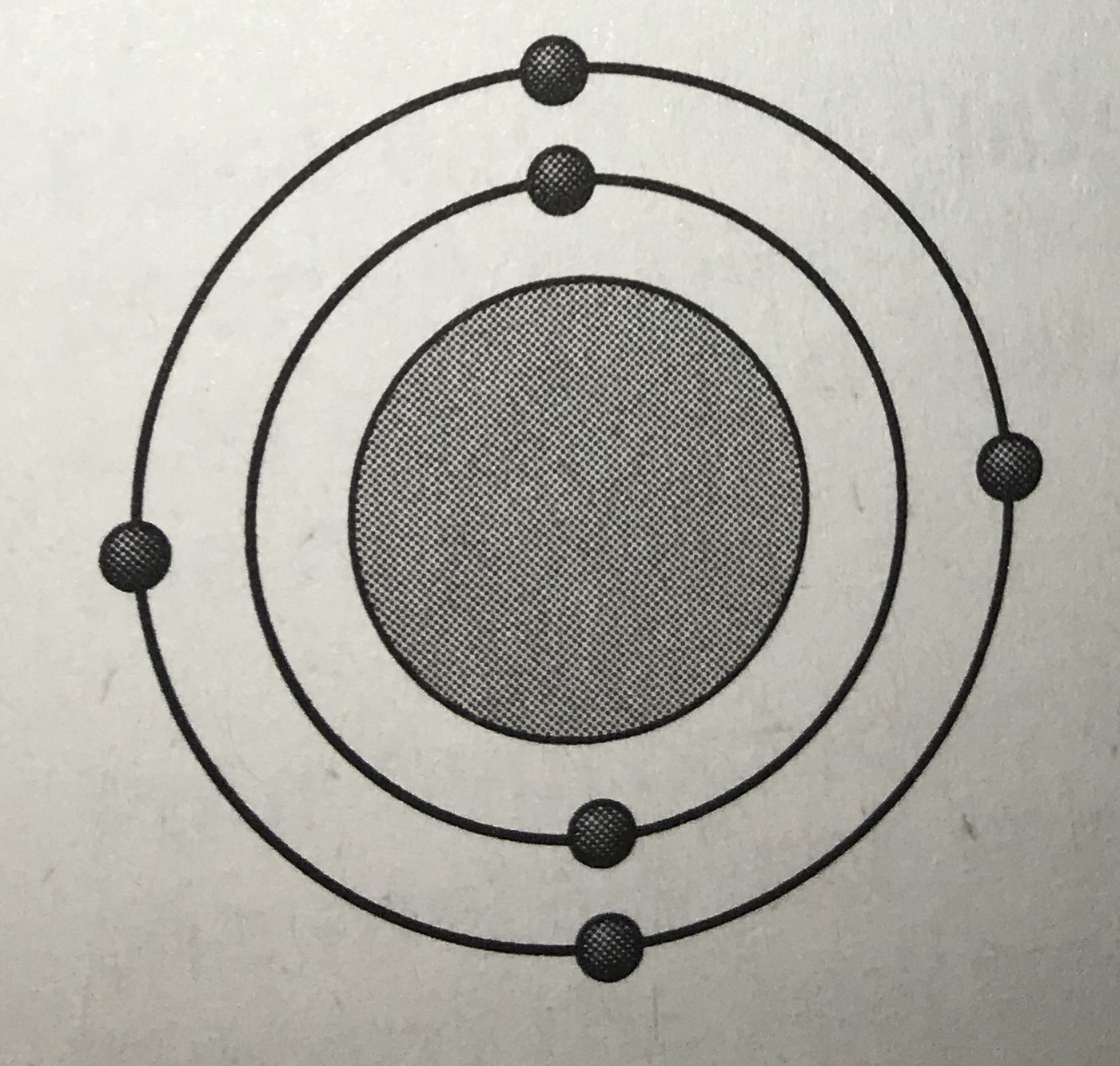
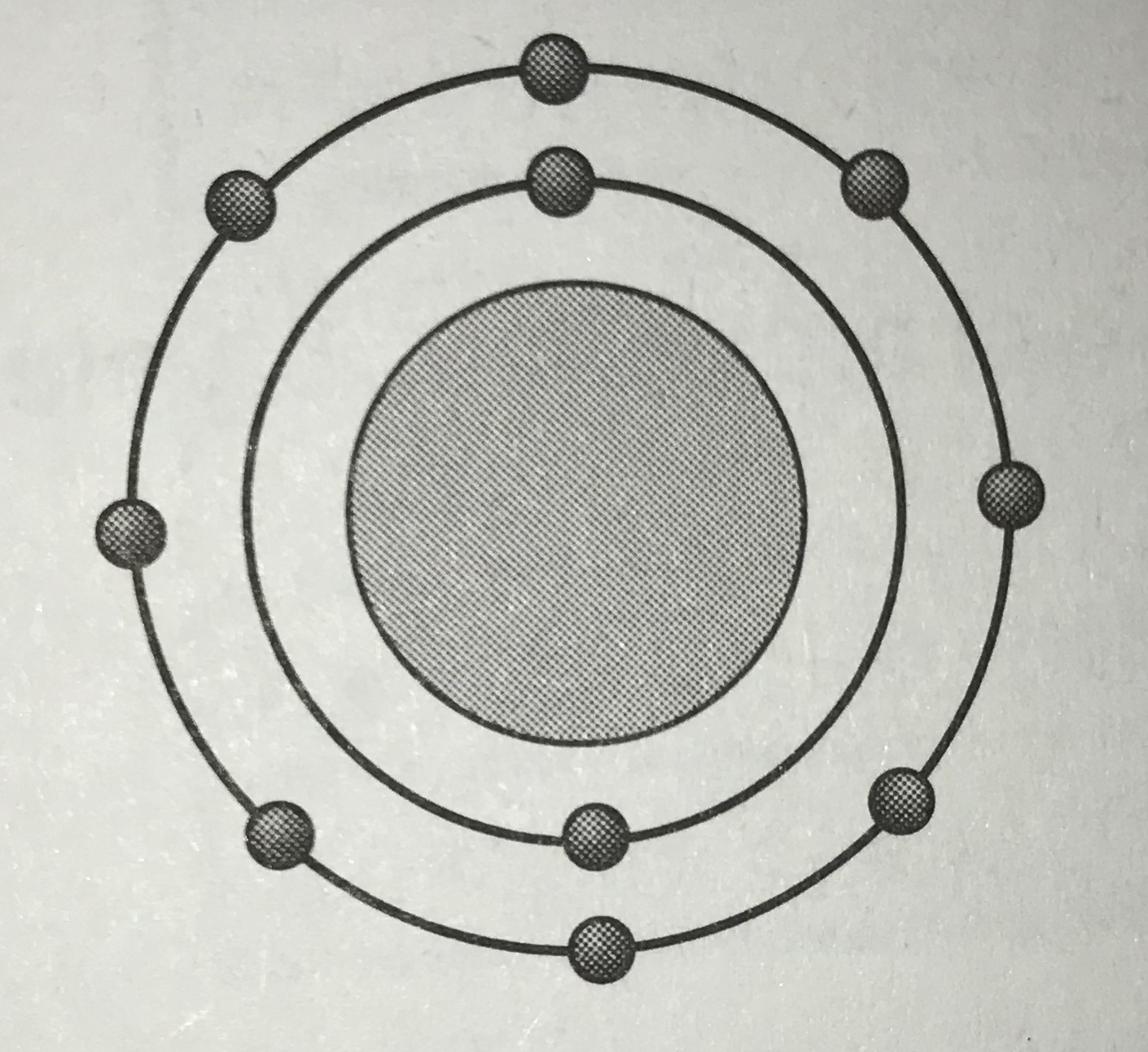
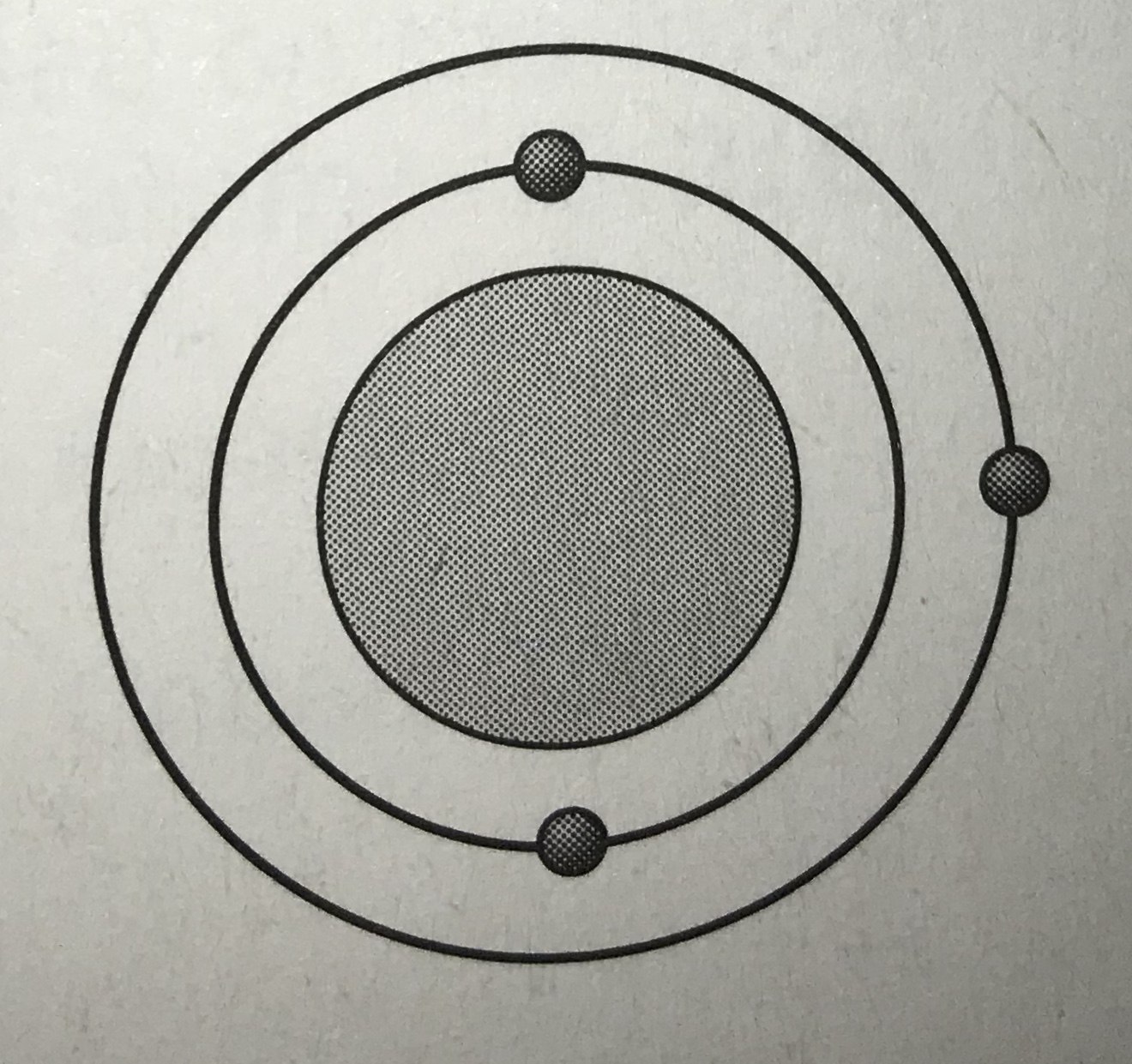
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
Which of the following characteristics is similar for fluorine and iodine?
Number of protons
Number of neutrons
Number of orbitals
Number of valence electrons
6.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
A group of students examines a Bohr model of an atom with 8 valence electrons. What conclusion can the students make about the chemical reactivity of the element?
The element is extremely reactive because the outer energy level is full
The element is nonreactive because the outer energy level is full
The element is somewhat reactive because the outer energy level is full
Additional information is necessary to determine reactivity
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
5 mins • 1 pt
The atomic number of an element is equal to its number of protons. Therefore, a logical conclusion is that the number of protons determines an element's
Mass and atomic weight
Number of stable isotopes
Identity
State of matter
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

The following two elements:
Have similar chemical properties and can be found in Group 7 of the periodic table
Do not have similar chemical properties and are not in the same group
Have similar chemical properties and can be found on opposite sides of the periodic table
Have similar chemical properties and can be found in Group 2 of the periodic table
Tags
NGSS.MS-PS1-1
Similar Resources on Wayground

11 questions
Identity and Reactivity
Quiz
•
8th Grade

11 questions
Identify
Quiz
•
8th Grade

12 questions
Metals, Nonmetals, Metalloids
Quiz
•
8th Grade

10 questions
Reactivity Chemistry
Quiz
•
8th - 10th Grade

12 questions
Periodic Table Families
Quiz
•
7th Grade

13 questions
Periodic Table
Quiz
•
8th Grade

12 questions
Matter Review (posttest)
Quiz
•
8th Grade

10 questions
Periodic table and reactivity
Quiz
•
8th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Physical and Chemical Properties
Quiz
•
8th Grade

20 questions
Counting Atoms Practice
Quiz
•
8th Grade

20 questions
Atomic Structure and Periodic Table
Quiz
•
7th Grade

20 questions
Chemical Reactions
Quiz
•
8th Grade

15 questions
Periodic Table of Elements
Quiz
•
8th Grade

15 questions
2.07: Aqueous Solutions
Quiz
•
6th - 8th Grade

20 questions
Pure substances and Mixtures
Quiz
•
8th Grade

10 questions
Chemistry: Elements, Compounds, and Mixtures Quiz
Passage
•
6th Grade
