
Chemical bonding
Quiz
•
Chemistry
•
10th Grade - University
•
Practice Problem
•
Medium
+1
Standards-aligned
Raneesh Konnola
Used 467+ times
FREE Resource
Enhance your content in a minute
20 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
An interaction that holds two atoms together called?
Chemical Bond
Covalent Bond
Ionic Bond
Metallic Bond
Tags
NGSS.HS-PS1-3
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Typically, atoms are more stable when they are
bonded together
apart from each other
Tags
NGSS.HS-PS1-4
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Why do atoms bond?
They typically don't bond
To add or take away energy levels
To have a full valance shell.
To have a full inner shell
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Valence electrons are
neutral (no charge)
found in the outer most energy level of the atom
equal to the number of protons
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

How many valence electrons in this structure?
Tags
NGSS.HS-PS1-1
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

How many more electrons are needed to fill an outermost energy level of a Carbon atom?
Tags
NGSS.HS-PS1-1
7.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which is the correct Lewis Structure for oxygen?

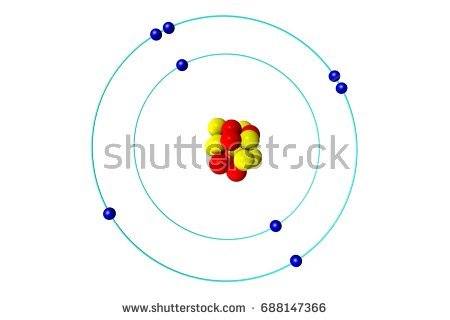
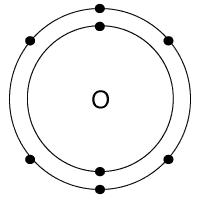
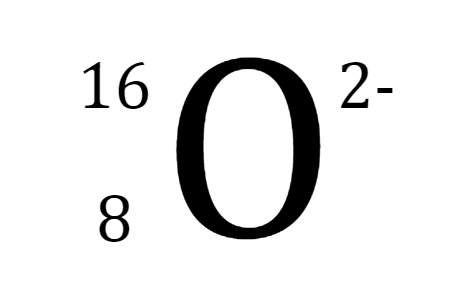
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

20 questions
Inorganic Chemistry: p - block elements
Quiz
•
University

21 questions
ELECTRON ARRANGEMENT
Quiz
•
10th - 12th Grade

20 questions
Electron Arrangement of Atoms
Quiz
•
9th - 12th Grade

20 questions
sec 2 lesson 4
Quiz
•
11th Grade

15 questions
Advance program 2020 yr 11 Chem Periodic Table Trends
Quiz
•
10th - 11th Grade

20 questions
Chemical Nomenclature
Quiz
•
10th - 11th Grade

16 questions
Electricity Quiz
Quiz
•
KG - University

21 questions
Which structure and particles?
Quiz
•
12th Grade
Popular Resources on Wayground

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
4:3 Model Multiplication of Decimals by Whole Numbers
Quiz
•
5th Grade

10 questions
The Best Christmas Pageant Ever Chapters 1 & 2
Quiz
•
4th Grade

12 questions
Unit 4 Review Day
Quiz
•
3rd Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

15 questions
Solving Equations with Variables on Both Sides Review
Quiz
•
8th Grade
Discover more resources for Chemistry

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Unit 6-Review The Mole
Quiz
•
11th - 12th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

148 questions
Fall TEKS Review Chemistry
Quiz
•
9th - 12th Grade

20 questions
Unit 5 - Chemical Reactions Refresh
Quiz
•
9th - 12th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade
