
Reaction Rates
Quiz
•
Chemistry
•
9th - 12th Grade
•
Practice Problem
•
Medium
Standards-aligned
Jennifer Scott-Burns
Used 231+ times
FREE Resource
Enhance your content in a minute
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A substance that increases speed of chemical reaction without being being changed is called:
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Increase in temperature of the reactants can do one of the following
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Grinding a seltzer tablet into powder increases the rate of reaction due to increased
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
This slows down or even stops a chemical reaction.
5.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
Which factors affect the rate of a reaction?
temperature
concentration
surface area
pressure
catalyst
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does a higher concentration increase the rate of reaction?
it increases the amount of reactants
it lowers the activation energy
it increases the energy of particle collisions
it increases the frequency of particle collisions
Tags
NGSS.HS-PS1-5
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which graph shows the effect of increasing temperature on the rate of reaction of calcium carbonate with dilute hydrochloric acid?
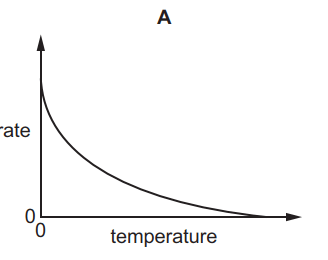
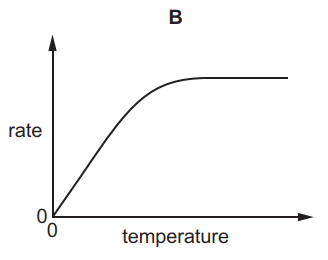
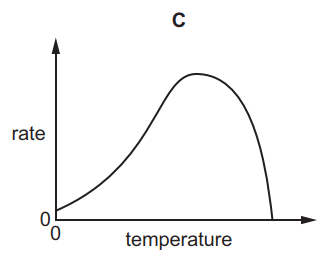
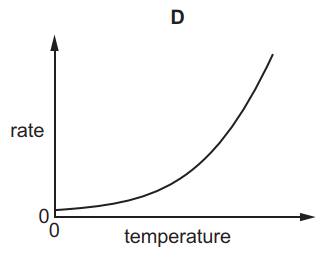
Tags
NGSS.HS-PS1-5
8.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

In which experiment is the rate of reaction between hydrochloric acid and calcium carbonate slowest?
Tags
NGSS.HS-PS1-5
Similar Resources on Wayground

10 questions
Chapter 26 - Understanding Intermolecular Forces
Quiz
•
11th Grade

11 questions
Matter and Atomic Structure
Quiz
•
10th - 12th Grade

10 questions
Amides
Quiz
•
1st Grade - University

10 questions
Chemistry Regent - June 2013 Q31-40
Quiz
•
9th - 12th Grade

10 questions
Redox Balancing Practice (acid solution)
Quiz
•
10th - 12th Grade

10 questions
Basic Kinetic Theory
Quiz
•
10th - 12th Grade

10 questions
Quiz 1: Mole Concept
Quiz
•
12th Grade - University

11 questions
Electrostatic Basics Inv 1&2 Review
Quiz
•
8th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade
