National 5 Chemistry - Unit 1 - Rates of Reactions
Quiz
•
Chemistry
•
7th Grade
•
Medium
Standards-aligned
Heather Lyall
Used 219+ times
FREE Resource
16 questions
Show all answers
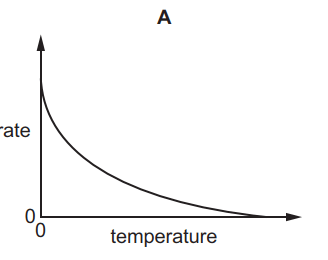
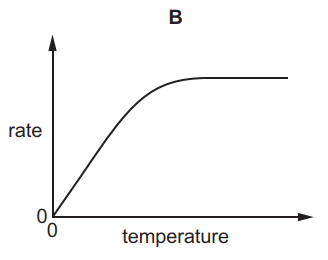
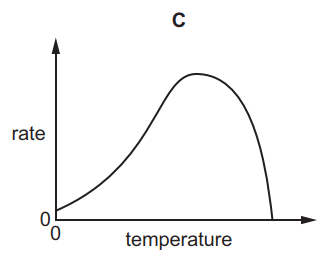
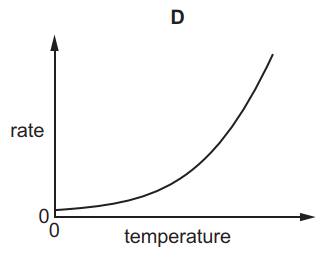
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt

A
B
C
D
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
A temperature increase causes the particles to ......
to slow down
move faster
collide higher
in the right order
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does a higher concentration increase the rate of reaction?
it increases the amount of reactants
it lowers the activation energy
it increases the energy of particle collisions
it increases the frequency of particle collisions
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt

What can be said about the green line?
it is a faster rate of reaction
It produces same volume of product as red line
Half the volume of reactants has been used
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which would not speed up a chemical reaction?
Using a large beaker
Increasing the concentration
Decreasing particle size
adding a catalyst
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which would have the slowest rate of reaction
Magnesium ribbon 1M HCl at 20 degrees
Magnesium powder 1M HCl at 20degrees
Magnesium ribbon 0.5M HCl at 10degrees
Magnesium powder 0.5M HCl at 10degrees
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Increasing the temperature gives particles more __________.
Time
Energy
Space
Frequency
Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

15 questions
ATS KS3 C3: Chemical Reactions
Quiz
•
7th Grade

15 questions
LEV KS3 Y7 Chemical Reactions
Quiz
•
7th - 8th Grade

15 questions
C4 Acid & metal/base/carbonate reactions
Quiz
•
7th - 10th Grade

14 questions
Chemical Bonds
Quiz
•
6th - 7th Grade

15 questions
7.5 Equilibrium
Quiz
•
6th - 10th Grade

20 questions
Hindenburg Vocab Review
Quiz
•
6th - 8th Grade

20 questions
KS4 Chemistry - Rates of Reaction
Quiz
•
7th - 11th Grade

13 questions
Chemistry
Quiz
•
7th - 8th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure and Periodic Table
Quiz
•
7th Grade

15 questions
2.07: Aqueous Solutions
Quiz
•
6th - 8th Grade

10 questions
Balancing Chemical Equations Challenge
Interactive video
•
6th - 10th Grade

22 questions
PHYSICAL AND CHEMICAL PROPERTIES
Quiz
•
7th - 9th Grade

20 questions
heat transfer
Quiz
•
7th Grade

20 questions
Physical vs. Chemical change
Quiz
•
6th - 7th Grade

20 questions
Counting Atoms Practice
Quiz
•
6th - 8th Grade

10 questions
Types of Mixtures and Their Properties
Interactive video
•
6th - 8th Grade