
Rate of reaction 1
Quiz
•
Chemistry
•
5th Grade
•
Practice Problem
•
Hard
Standards-aligned
Used 617+ times
FREE Resource
Enhance your content in a minute
13 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the meaning of the rate of reaction?
Decrease in amount of product
Decrease in amount of product against time
Increase in amount of products against time
Increase in amount of reactants against time
Tags
NGSS.HS-PS1-5
2.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which unit is correct for the rate of reaction?
g mol-1
g min-1
mol dm-3
kJ mol-1
Tags
NGSS.HS-PS1-5
3.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which of the following has the lowest rate of reaction?
Combustion of ethanol
Fermentation of glucose
Oxidation of magnesium
Precipitation of silver chloride
Tags
NGSS.HS-PS1-5
4.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
Which reactions has the highest rate of reaction?
Rusting of water pipe
Photosynthesis in green plant
Burning of a small piece of charcoal in the air
Formation of stalactites and stalagmites
Tags
NGSS.HS-PS1-5
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which process has the highest rate of reaction?
Rusting
Respiration
Combustion
Photosynthesis
Tags
NGSS.HS-PS1-5
6.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
The following equation represents a chemical equation.
CaCO3 + 2HCl --> CaCl2 + CO2 + H2O
Which graph shows the correct change in mass of reactant used in excess against time?


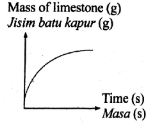
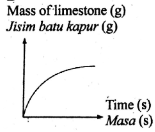
Tags
NGSS.HS-PS1-7
7.
MULTIPLE CHOICE QUESTION
2 mins • 1 pt
The rate of reaction for the decomposition of hydrogen peroxide decreases with time because
product of reaction decreases
temperature of hydrogen peroxide decreases
volume of hydrogen peroxide decreases
concentration of hydrogen peroxide decreases
Tags
NGSS.HS-PS1-5
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

15 questions
Relative atomic mass & relative molecular mass
Quiz
•
1st - 10th Grade

10 questions
Michigan!
Quiz
•
KG - University

15 questions
States of Matter
Quiz
•
5th - 7th Grade

12 questions
Bones
Quiz
•
1st - 12th Grade

12 questions
The negative in French
Quiz
•
KG - 7th Grade

11 questions
WHMIS Review
Quiz
•
1st - 12th Grade

15 questions
Chemical Magic
Quiz
•
4th - 6th Grade

17 questions
8th grade science STAAR review 1
Quiz
•
KG - University
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

20 questions
Making Inferences
Quiz
•
5th Grade

10 questions
Exploring Europe: Geography, History, and Culture
Interactive video
•
5th - 8th Grade

10 questions
Simplifying Fractions
Quiz
•
5th Grade

10 questions
Charlie Brown's Thanksgiving Adventures
Interactive video
•
2nd - 5th Grade

